Nakashima Medical Co., Ltd. – Manufacturing of medical devices, such as artificial joints
- Company Name: Nakashima Medical Co., Ltd.
- State/Prefecture: Okayama
- City/Town/Village: Okayama
- Street: 688-1 Jotokitagata, Higashi-ku
- Country: Japan
- Zip/Postal Code: 709-0625
- Phone: +81-86-279-6278
- Website: http://www.medical.nakashima.co.jp/en/
- Contact: Fax +81-86-279-9510
- Listed: 05/28/2014 5:27 am
- Expires: This ad has expired
Nakashima Medical entered into the new field of “joint replacement” by making use of the processing technology it has applied to marine equipment for over 80 years. After the company was spun off in September 2008, we structured ourselves to intricately respond to doctor’s needs as “Nakashima Medical Co., Ltd.”
Products:
Joint Prosthesis
- Shoulder joint prosthesis:
 Shoulder joint prosthesis are mainly comprised of a scapula side and humerus side. Major indications are osteoarthritis and rheumatoid arthritis. Nakashima Medical offers shoulder joint prosthesis specialized for rheumatoid arthritis for only the humerus side.
Shoulder joint prosthesis are mainly comprised of a scapula side and humerus side. Major indications are osteoarthritis and rheumatoid arthritis. Nakashima Medical offers shoulder joint prosthesis specialized for rheumatoid arthritis for only the humerus side.
- Elbow joint prosthesis:
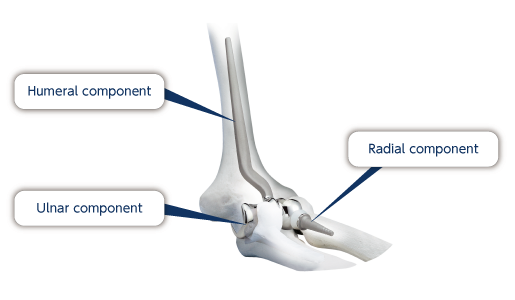 Elbow joint prosthesis are mainly comprised of humeral and ulnar components. They are reconstructed by sliding the articular surfaces of these two components. Depending on the product, we can offer a radial component that enables the elbow to bend and stretch naturally.
Elbow joint prosthesis are mainly comprised of humeral and ulnar components. They are reconstructed by sliding the articular surfaces of these two components. Depending on the product, we can offer a radial component that enables the elbow to bend and stretch naturally.
- Finger joint prosthesis:
 There are different kinds of joint prosthesis for each of the finger’s three joint. Finger joint prosthesis are comprised of two parts, i.e., a distal phalanx side and middle phalanx side for DIP, middle phalanx side and proximal phalanx side for PIP, and proximal phalanx side and metacarpal side for MP.
There are different kinds of joint prosthesis for each of the finger’s three joint. Finger joint prosthesis are comprised of two parts, i.e., a distal phalanx side and middle phalanx side for DIP, middle phalanx side and proximal phalanx side for PIP, and proximal phalanx side and metacarpal side for MP.
- Hip joint prosthesis:
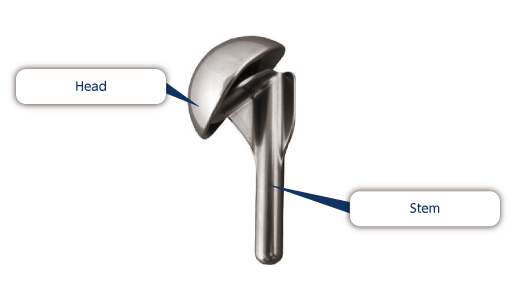
 Hip joint prosthesis are mainly comprised of a shell cup, shell liner, femoral head and stem. Hip joints are reconstructed by sliding the articular surfaces of the femoral head and shell liner. There are two ways of anchoring joint prosthesis, with bone cement or without bone cement.
Hip joint prosthesis are mainly comprised of a shell cup, shell liner, femoral head and stem. Hip joints are reconstructed by sliding the articular surfaces of the femoral head and shell liner. There are two ways of anchoring joint prosthesis, with bone cement or without bone cement.
- Knee joint prosthesis:
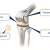
 Knee joint prosthesis are mainly comprised of four components: femoral component, tibial base plate, tibial insert and patella component. Knee joints are reconstructed by sliding the articular surfaces of femoral components and polyethylene tibial plates.
Knee joint prosthesis are mainly comprised of four components: femoral component, tibial base plate, tibial insert and patella component. Knee joints are reconstructed by sliding the articular surfaces of femoral components and polyethylene tibial plates.
- Ankle joint prosthesis:
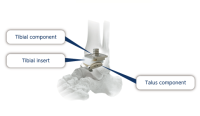
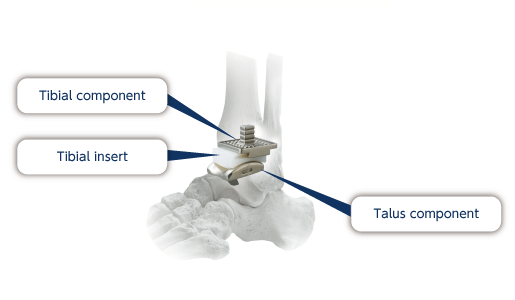 Ankle joint prosthesis are comprised of a talus component, tibial component and tibial insert. Ankle joints are reconstructed by sliding the articular surfaces of the talus component and tibial component..
Ankle joint prosthesis are comprised of a talus component, tibial component and tibial insert. Ankle joints are reconstructed by sliding the articular surfaces of the talus component and tibial component..
Trauma Devices
- Intramedullary nails:
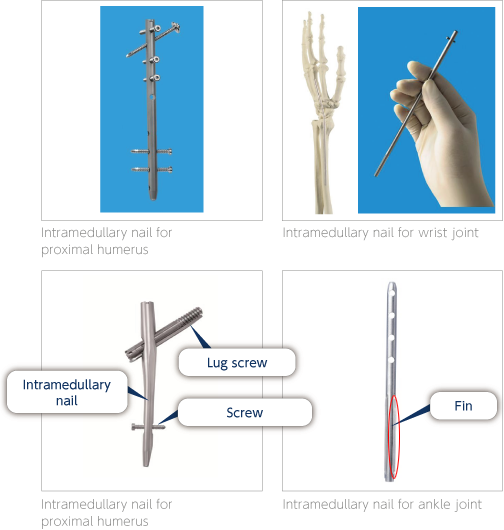 Intramedullary nails are used for the surgical treatment of fractures and rheumatoid arthritis. They are mainly composed of an intramedullary nail and some screws. Thick metal lug screws are sometimes used to ensure stronger anchoring. The intramedullary nail inserted in the center of the bone supports the bone and can tightly anchor the targeted area. The lug screws facilitate bone adhesion by applying moderate compression on a reduced area. Screws are used for immobilizing the intramedullary nail in the bone with the main purpose of preventing sinking and rotation. In order to prevent rotation, Nakashima Medical has developed a nail with fins that immobilizes the bone with greater force.
Intramedullary nails are used for the surgical treatment of fractures and rheumatoid arthritis. They are mainly composed of an intramedullary nail and some screws. Thick metal lug screws are sometimes used to ensure stronger anchoring. The intramedullary nail inserted in the center of the bone supports the bone and can tightly anchor the targeted area. The lug screws facilitate bone adhesion by applying moderate compression on a reduced area. Screws are used for immobilizing the intramedullary nail in the bone with the main purpose of preventing sinking and rotation. In order to prevent rotation, Nakashima Medical has developed a nail with fins that immobilizes the bone with greater force.
- Plate and screw system:
 A plate and screw anchoring system is used for the surgical treatment of fractures, bone dislocation and osteotomy. The plate supports the damaged bone by being placed on the fracture site. The plate and screw ensure rigid anchoring with the bone after surgery. Nakashima Medical offers a wide range of plate and screw systems to address a large variety of fracture sites. Locking screws that allow the rigid anchoring are available. Surgeons can choose the best screws for each case.
A plate and screw anchoring system is used for the surgical treatment of fractures, bone dislocation and osteotomy. The plate supports the damaged bone by being placed on the fracture site. The plate and screw ensure rigid anchoring with the bone after surgery. Nakashima Medical offers a wide range of plate and screw systems to address a large variety of fracture sites. Locking screws that allow the rigid anchoring are available. Surgeons can choose the best screws for each case.
ISO certification
Nakashima Medical has obtained “ISO 13485” accreditation.
“ISO 13485” is an international standard regarding the quality assurance of medical devices. It was created to establish quality management standards for global medical device industry by adding the requirements of medical devices to “ISO 9001.”
Nakashima Medical will continue to pursue safe high quality products that meet world standards by maintaining our “ISO 13485” accreditation.